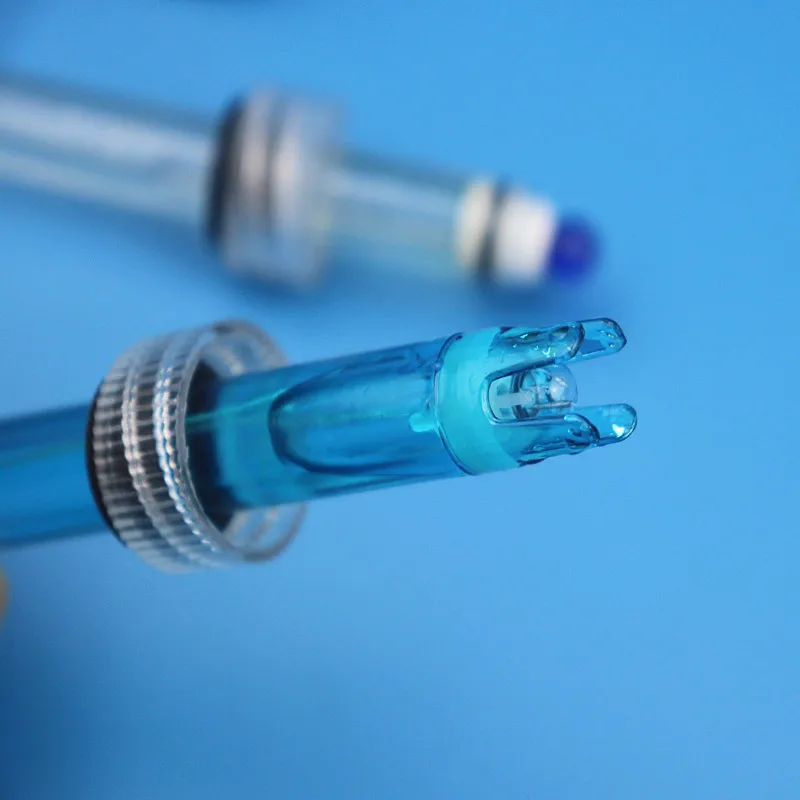
This electrode is a non - refillable composite electrode with a plastic shell, combining a glass electrode and a reference electrode. It is a pH measurement element, used to measure the activity of hydrogen ions (pH value) in aqueous solutions. It is widely applied in places where acid - base detection is required in the chemical industry, pharmaceutical industry, dye industry, and scientific research.
Measurement Range: 0 - 14 pH
Measurement Temperature: 0 - 60°C
Zero Potential: 7 ± 0.5 pH (25°C)
Percentage Theoretical Slope: (PTS) ≥ 98.5% (25°C)
Internal Resistance: ≤ 250 MΩ (25°C)
Alkali Error: 0.2 pH (1 mol/L Na⁺, pH 14) (25°C)
Response Time: ≤ 1 minute
Before measurement, the electrode must be calibrated with standard buffer solutions of known pH values. For more accurate results, the known pH value should be reliable and as close as possible to the measured value.
After removing the electrode protective cover, ensure that the sensitive glass bulb inside the plastic protection grid does not come into contact with hard objects. Any damage or abrasion will render the electrode ineffective.
After measurement, put the electrode protective cover back on in a timely manner. A small amount of 3.3 mol/L potassium chloride solution should be placed inside the protective cover to keep the electrode bulb moist.
The lead - out ends of the electrode must be kept clean and dry. Absolutely prevent short - circuiting of the two output ends, otherwise the measurement results will be inaccurate or the electrode will fail.
The electrode should be used with an acidimeter with a relatively high input impedance (≥ 1012 Ω) to maintain the good characteristics of the electrode.
The electrode should avoid being immersed in distilled water, protein solutions, or acidic fluoride solutions for a long time, and prevent contact with organic silicone grease.
After long - term use, if the slope of the electrode is slightly reduced, the lower end of the electrode can be soaked in 4% HF (hydrofluoric acid) for 3 - 5 seconds, rinsed with distilled water, and then soaked in potassium chloride solution to rejuvenate it.
If the measured solution contains substances that easily contaminate the sensitive bulb and block the liquid junction, causing the electrode to become passivated (characterized by a reduced sensitivity gradient and inaccurate readings), the electrode should be cleaned with an appropriate solution according to the nature of the contaminant to rejuvenate it.
For organic solvents that can dissolve polycarbonate resin, select a pH electrode whose glass shell model is compatible with the electrode (such as the 65 - 1Q9 type composite electrode).
Note: When choosing a cleaning agent, if the cleaning solution can dissolve polycarbonate resin (such as carbon tetrachloride, trichloroethylene, tetrahydrofuran, etc.), it may dissolve the polycarbonate resin and coat the sensitive glass bulb, rendering the electrode ineffective. Use with caution!